Introduction: Outside of a clinical trial setting, ibrutinib is the most well-characterized of the covalent Bruton's tyrosine kinase inhibitors used in the treatment of patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) in the first-line (1L) setting. However, the impact of ibrutinib dose adjustment on clinical outcomes remains to be defined. In this real-world effectiveness study, we compared time to next treatment (TTNT) as a proxy for disease progression for dose adjustment and standard dose treatment strategies in patients with CLL/SLL receiving 1L single-agent IBR.
Methods: This study utilized the Komodo Health payer-complete dataset, derived from U.S. health insurance claims from >150 private insurers covering >160 million individuals from 2015-2023; 53% of these patients are from community centers. Adult patients with CLL/SLL were included if they initiated 1L single-agent IBR 420 mg/day between March 2016 - April 2023 (IBR initiation date = index date; 1L single-agent IBR was defined as no other CLL/SLL treatment in the 12-month baseline period or within 28 days after index). A target trial emulation (TTE) approach was utilized to compare TTNT, defined as the time between the index date to the initiation of a next line of therapy (LOT), for two treatment strategies: 1) Dose adjustment strategy, defined as a lead-in IBR treatment at 420mg/day for 3-12 months, followed by dose adjustment to <420mg/day until next LOT or IBR discontinuation; 2) Standard dose strategy, defined as a continuous IBR treatment at 420mg/day without any dose adjustment for >12 months until next LOT or IBR discontinuation. The TTE approach minimizes immortal time bias in a naïve observational analysis as patients in the dose adjustment cohort cannot initiate next LOT before dose adjustment occurs [Hernan et al. Am J Epidemiol. 2016]. To explicitly emulate a target trial, two clones were created for each patient, with one clone contributing person-time and event to each treatment strategy of interest, respectively. A clone was artificially censored when the actual treatment path deviated from the assigned treatment strategy. Selection bias due to artificial censoring was corrected using time-varying inverse probability of censoring weights (IPCW), accounting for post-index time-varying clinical factors related to IBR dose adjustment or IBR discontinuation. TTNT hazard ratio (HR; robust 95% confidence interval [CI]) was estimated using an IPCW-weighted pooled logistic regression model. Alternative lead-in periods with IBR standard dosing (3-6 and 3-9 months) were also evaluated. A subgroup analysis was conducted among patients with high cardiovascular (CV) risk, defined as having a preexisting CV comorbidity or a high CHA 2DS 2-Vasc risk score.
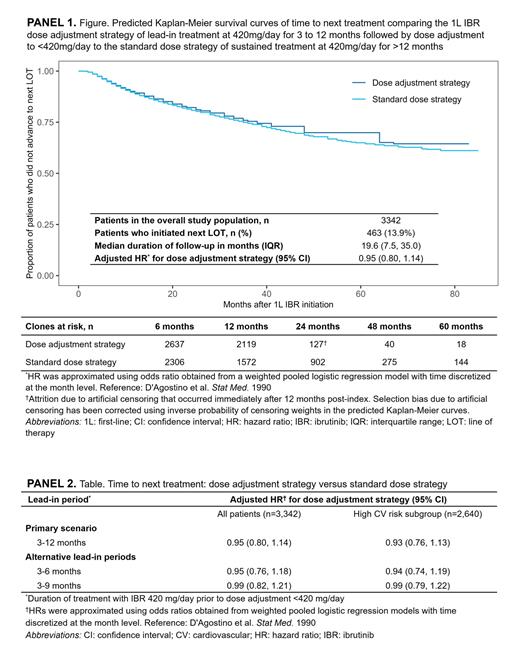
Results: 3,342 adults initiated 1L single-agent IBR at 420 mg/day. Mean (standard deviation [SD]) age was 67.5 (10.6) years, 62% were male, and mean (SD) Quan-Charlson Comorbidity Index score was 3.0 (1.5). The median (interquartile range) follow-up duration was 19.6 (7.5, 35.0) months. During the study period, 463 patients (14%) initiated a subsequent LOT. The high CV risk subgroup consisted of 2,640 (79%) patients. In the overall study population, 608 (18%) patients experienced a dose adjustment after the index date. Of those, the dose adjustment occurred ≤3 months, ≤6 months, and ≤12 months post-index in 159 (26%), 300 (49%), and 440 (72%) patients, respectively, with dose used at the first reduction of 70 mg, 140 mg, or 280 mg in 4 (1%), 85 (14%), and 519 (85%) patients, respectively. The dose adjustment strategy (420 mg/day for 3-12 months followed by a reduced dose) was not associated with increased risk of initiating next LOT (adjusted HR [95% CI] 0.95 [0.80, 1.14]) compared to the continuous standard dose strategy; results for the alternative lead-in periods and the high CV risk subgroup were consistent with the primary scenario.
Conclusions: In this real-world comparative effectiveness study with the majority of patients from community centers, dose adjustment of 1L single-agent IBR treatment for CLL/SLL is not associated with an increased risk of initiating next LOT when compared to continuous standard dose treatment. These findings suggest that a flexible dosing approach with IBR may be an effective option to maintain an optimal balance of clinical therapeutic efficacy and safety for patients with CLL/SLL in the 1L setting.
Disclosures
Ghosh:Roche NHL solutions panel: Membership on an entity's Board of Directors or advisory committees; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Kite Pharma, Beigene, Incyte, Lava Therapeutics, Incyte, Roche/Genentech, Novartis, Loxo Oncology, AbbVie, Genmab, Adaptive Biotech, ADC Therapeutics: Consultancy; TG Therapeutics, Genentech/Roche, Bristol Myers Squibb, Gilead, Morphosys, AbbVie, Pharmacyclics: Research Funding; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Kite Pharma, Beigene, Incyte, Lava Therapeutics, Incyte, Roche/Genentech, Novartis, Loxo Oncology, AbbVie, Genmab, Adaptive Biotech, ADC Therapeutics, Morp: Honoraria; AstraZeneca, Janssen, Pharmacyclics, Kite pharma, BMS, Epizyme: Speakers Bureau. Wang:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company. Ding:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company. He:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company. Bokun:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company. Mavani:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company. Qureshi:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company. Rogers:Pharmacyclics: Consultancy; Novartis: Research Funding; Loxo@Lilly: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; Genentech: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal